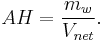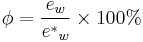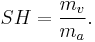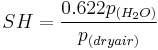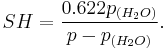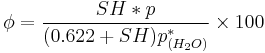Humidity
Humidity is a term for the amount of water vapor in the air, and can refer to any one of several measurements of humidity. Formally, humid air is not "moist air" but a mixture of water vapor and other constituents of air, and humidity is defined in terms of the water content of this mixture, called the Absolute humidity.[1] In everyday usage, it commonly refers to relative humidity, expressed as a percent in weather forecasts and on household humidistats; it is so called because it measures the current absolute humidity relative to the maximum. Specific humidity is a ratio of the water vapor content of the mixture to the total air content (on a mass basis). The water vapor content of the mixture can be measured either as mass per volume or as a partial pressure, depending on the usage.
In meteorology, humidity indicates the likelihood of precipitation, dew, or fog. High relative humidity reduces the effectiveness of sweating in cooling the body by reducing the rate of evaporation of moisture from the skin. This effect is calculated in a heat index table, used during summer weather.
Contents |
Types
Absolute humidity
If all the water vapor in one cubic meter of air were condensed into a container, the mass of the water in the container could be measured to determine absolute humidity. The amount of water vapor in that cube of air is the absolute humidity of that cubic meter of air. Absolute humidity, on a volume basis, is the mass of water vapor, 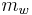 , per cubic meter of total moist air,
, per cubic meter of total moist air, 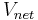 :
:
Absolute humidity ranges from 0 grams per cubic meter in dry air to 30 grams per cubic meter (0.22 ounce per cubic foot) when the vapor is saturated at 30 °C.[1] (See also Absolute Humidity table)
The absolute humidity changes as air pressure changes. This is very inconvenient for chemical engineering calculations, e.g. for clothes dryers, where temperature can vary considerably. As a result, absolute humidity is generally defined in chemical engineering as mass of water vapor per unit mass of dry air, also known as the mass mixing ratio (see below), which is much more rigorous for heat and mass balance calculations. Mass of water per unit volume as in the equation above would then be defined as volumetric humidity. Because of the potential confusion, British Standard BS 1339 (revised 2002) suggests avoiding the term "absolute humidity". Units should always be carefully checked. Most humidity charts are given in g/kg or kg/kg, but any mass units may be used.
The field concerned with the study of physical and thermodynamic properties of gas-vapor mixtures is named Psychrometrics.
Relative humidity
Relative humidity is a term used to describe the amount of water vapor in a mixture of air and water vapor. It is defined as the ratio of the partial pressure of water vapor in the air-water mixture to the saturated vapor pressure of water at those conditions. The relative humidity of air depends not only on temperature but also on pressure of the system of interest.
Relative humidity is normally expressed as a percentage and is calculated by using the following equation:[2]
Relative humidity is an important metric used in weather forecasts and reports, as it is an indicator of the likelihood of precipitation, dew, or fog. In hot summer weather, a rise in relative humidity also increases the apparent temperature to humans (and other animals) by hindering the evaporation of perspiration from the skin as the relative humidity rises. For example, according to the Heat Index, a relative humidity of 75% at 80°F (27°C) would feel like 83.574°F ±1.3 °F (28.652°C ±1.7 °C) at ~44% relative humidity.[3]
Specific humidity
Specific humidity is the ratio of water vapor to dry air in a particular mass, and is sometimes referred to as humidity ratio. Specific humidity ratio is expressed as a ratio of grams of water vapor, 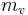 , per kilogram of dry air
, per kilogram of dry air 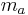 [4] .
[4] .
That ratio is defined as:
Specific humidity can be expressed in other ways including:
or:
Using the definition of specific humidity, the relative humidity can be expressed as
However, specific humidity is also defined as the ratio of water vapor to the total mass of the system in meteorology.[5] "Mixing ratio" is used to name the definition in this section beginning.[6]
Measurement
There are various devices used to measure and regulate humidity. A device used to measure humidity is called a psychrometer or hygrometer. A humidistat is used to regulate the humidity of a building with a dehumidifier. These can be analogous to a thermometer and thermostat for temperature control.
Humidity is also measured on a global scale using remotely placed satellites. These satellites are able to detect the concentration of water in the troposphere at altitudes between 4 and 12 kilometers. Satellites that can measure water vapor have sensors that are sensitive to infrared radiation. Water vapor specifically absorbs and re-radiates radiation in this spectral band. Satellite water vapor imagery plays an important role in monitoring climate conditions (like the formation of thunderstorms) and in the development of future weather forecasts.
Climate
While humidity itself is a climate variable, it also interacts strongly with other climate variables. The humidity is affected by winds and by rainfall. At the same time, humidity affects the energy budget and thereby influences temperatures in two major ways. First, water vapor in the atmosphere contains "latent" energy. During transpiration or evaporation, this latent heat is removed from surface liquid, cooling the earth's surface. This is the biggest non-radiative cooling effect at the surface. It compensates for roughly 70% of the average net radiative warming at the surface. Second, water vapor is the most important of all greenhouse gases. Water vapor, like a green lens that allows green light to pass through it but absorbs red light, is a "selective absorber". Along with other greenhouse gases, water vapor is transparent to most solar energy, as you can literally see. But it absorbs the infrared energy emitted (radiated) upward by the earth's surface, which is the reason that humid areas experience very little nocturnal cooling but dry desert regions cool considerably at night. This selective absorption causes the greenhouse effect. It raises the surface temperature substantially above its theoretical radiative equilibrium temperature with the sun, and water vapor is the cause of more of this warming than any other greenhouse gas.
The most humid cities on earth are generally located closer to the equator, near coastal regions. Cities in South and Southeast Asia are among the most humid, such as Kolkata, Chennai and Cochin in India, the cities of Manila in the Philippines and Bangkok in Thailand: these places experience extreme humidity during their rainy seasons combined with warmth giving the feel of a lukewarm sauna.[7] Darwin, Australia experiences an extremely humid wet season from December to April. Shanghai and Hong Kong in China also have an extreme humid period in their summer months. Kuala Lumpur and Singapore have very high humidity all year round because of their proximity to water bodies and the equator and overcast weather. Perfectly clear days are dependent largely upon the season in which one decides to travel. During the South-west and North-east Monsoon seasons (respectively, late May to September and November to March), expect heavy rains and a relatively high humidity post-rainfall. Outside the monsoon seasons, humidity is high (in comparison to countries North of the Equator), but completely sunny days abound. In cooler places such as Northern Tasmania, Australia, high humidity is experienced all year due to the ocean between mainland Australia and Tasmania. In the summer the hot dry air is absorbed by this ocean and the temperature rarely climbs above 35 degrees Celsius.
In the United States the most humid cities, strictly in terms of relative humidity, are Forks and Olympia, Washington.[8] This fact may come as a surprise to many, as the climate in this region rarely exhibits the discomfort usually associated with high humidity. Dew points are typically much lower on the West Coast than on the East. Because high dew points play a more significant role than relative humidity in the discomfort created during humid days, the air in these western cities usually does not feel "humid".
The highest dew points in the US are found in coastal Florida and Texas. When comparing Key West and Houston, two of the most humid cities from those states, coastal Florida seems to have the higher dew points on average. However, Houston lacks the coastal breeze present in Key West, and, as a much larger city, it suffers from the urban heat island effect.[9] A dew point of 86 degrees Fahrenheit was recorded in southern Minnesota on July 23, 2005, though dew points over 80 degrees Fahrenheit are rare there.[10] The US city with the lowest annual humidity is Las Vegas, Nevada, averaging 39% for a high and 21% as a low.[11]
Air density and volume
Humidity depends on water vaporization and condensation, which, in turn, mainly depends on temperature. Therefore, when applying more pressure to a gas saturated with water, all components will initially decrease in volume approximately according to the ideal gas law. However, some of the water will condense until returning to almost the same humidity as before, giving the resulting total volume deviating from what the ideal gas law predicted. Conversely, decreasing temperature would also make some water condense, again making the final volume deviating from predicted by the ideal gas law. Therefore, gas volume may alternatively be expressed as the dry volume, excluding the humidity content. This fraction more accurately follows the ideal gas law. On the contrary the saturated volume is the volume a gas mixture would have if humidity was added to it until saturation (or 100% relative humidity).
Humid air is less dense than dry air because a molecule of water (M ≈ 18 u ) is less massive than either a molecule of nitrogen (M ≈ 28) or a molecule of oxygen (M ≈ 32). About 78% of the molecules in dry air are nitrogen (N2). Another 21% of the molecules in dry air are oxygen (O2). The final 1% of dry air is a mixture of other gases.
For any gas, at a given temperature and pressure, the number of molecules present in a particular volume is constant – see ideal gas law. So when water molecules (vapor) are introduced into that volume of dry air, the number of air molecules in the volume must decrease by the same number, if the temperature and pressure remain constant. (The addition of water molecules, or any other molecules, to a gas, without removal of an equal number of other molecules, will necessarily require a change in temperature, pressure, or total volume; that is, a change in at least one of these three parameters. If temperature and pressure remain constant, the volume increases, and the dry air molecules that were displaced will initially move out into the additional volume, after which the mixture will eventually become uniform through diffusion.) Hence the mass per unit volume of the gas—its density—decreases. Isaac Newton discovered this phenomenon and wrote about it in his book Opticks.[12]
Effects
Animals and plants
Humidity is one of the fundamental abiotic factors that defines any habitat, and is a determinant of which animals and plants can thrive in a given environment.[13]
The human body dissipates heat by a perspiration and evaporation. Heat convection to the surrounding air, and thermal radiation are the primary modes of heat transport from the body. Under conditions of high humidity, the rate of evaporation of sweat from the skin decreases. Also, if the atmosphere is as warm as or warmer than the skin during times of high humidity, blood brought to the body surface cannot dissipate heat by conduction to the air, and a condition called hyperpyrexia results. With so much blood going to the external surface of the body, relatively less goes to the active muscles, the brain, and other internal organs. Physical strength declines, and fatigue occurs sooner than it would otherwise. Alertness and mental capacity also may be affected, resulting in heat stroke or hyperthermia.
Human comfort
Humans are sensitive to humid air because the human body uses evaporative cooling as the primary mechanism to regulate temperature. Under humid conditions, the rate at which perspiration evaporates on the skin is lower than it would be under arid conditions. Because humans perceive the rate of heat transfer from the body rather than temperature itself,[6] we feel warmer when the relative humidity is high than when it is low.
Some people experience difficulty breathing in high humidity environments. Some cases may possibly be related to respiratory conditions such as asthma, while others may be the product of anxiety. Sufferers will often hyperventilate in response, causing sensations of numbness, faintness, and loss of concentration, among others.[14]
Air conditioning works by reducing humidity in summer. In winter, heating cold outdoor air can decrease relative humidity levels indoor to below 30%, leading to discomfort such as dry skin and excessive thirst.
Electronics
Many electronic devices have humidity specifications, for example, 5% to 95%. At the top end of the range, moisture may increase the conductivity of permeable insulators leading to malfunction. Too low humidity may make materials brittle. A particular danger to electronic items, regardless of the stated operating humidity range, is condensation. When an electronic item is moved from a cold place (e.g., garage, car, shed, an air conditioned space in the tropics) to a warm humid place (house, outside tropics), condensation may coat circuit boards and other insulators, leading to short circuit inside the equipment. Such short circuits may cause substantial permanent damage if the equipment is powered on before the condensation has evaporated. A similar condensation effect can often be observed when a person wearing glasses comes in from the cold. It is advisable to allow electronic equipment to acclimatise for several hours, after being brought in from the cold, before powering on. Some electronic devices can detect such a change and indicate, when plugged in and usually with a small droplet symbol, that they cannot be used until the risk from condensation has passed. In situations where time is critical, increasing air flow through the device's internals when, such as removing the side panel from a PC case and directing a fan to blow into the case will reduce significantly the time needed to acclimatise to the new environment.
On the opposite, very low humidity level favors the buildup of static electricity, which may result in spontaneous shutdown of computers when discharges occur. Apart from spurious erratic function, electrostatic discharges can cause dielectric breakdown in solid state devices, resulting in irreversible damage. Data centers often monitor relative humidity levels for these reasons.
Building construction
Traditional building designs typically had weak insulation, and it allowed air moisture to flow freely between the interior and exterior. The energy-efficient, heavily-sealed architecture introduced in the 20th century also sealed off the movement of moisture, and this has resulted in a secondary problem of condensation forming in and around walls, which encourages the development of mold and mildew. Additionally, buildings with foundations not properly sealed will allow water to flow through the walls due to capillary action of pores found in masonry products. Solutions for energy-efficient buildings that avoid condensation are a current topic of architecture.
See also
References
- ^ Wyer, S.S., "A treatise on producer-gas and gas-producers", (1906) The Engineering and Mining Journal, London, p.23
- ^ Perry, R.H. and Green, D.W, Perry's Chemical Engineers' Handbook (7th Edition), McGraw-Hill, ISBN 0-07-049841-5 , Eqn 12-7
- ^ http://en.wikipedia.org/wiki/Heat_index#Formula
- ^ Cengel, Yunus and Boles, Michael, Thermodynamics: An Engineering Approach, 1998, 3rd edition, McGraw-Hill, pp. 725–726
- ^ AMS Glossary: specific humidity
- ^ http://amsglossary.allenpress.com/glossary/search?id=mixing-ratio1
- ^ BBC – Weather Centre – World Weather – Average Conditions – Bangkok
- ^ What Is The Most Humid City In The U.S.? | KOMO-TV – Seattle, Washington | News Archive
- ^ Answers: Is Florida or Texas more humid: September 3,2003
- ^ High Dew Point Temperatures: July 23, 2005
- ^ http://www.cityrating.com/relativehumidity.asp
- ^ Isaac Newton (1704). Opticks. Dover. ISBN 9780486602059. http://books.google.com/books?id=iTpXLrPR2TQC&printsec=frontcover&dq=isaac+newton+optics.
- ^ C.Michael Hogan. 2010. Abiotic factor. Encyclopedia of Earth. eds Emily Monosson and C. Cleveland. National Council for Science and the Environment. Washington DC
- ^ "I have trouble breathing in humidity - Lung & Respiratory Disorders / COPD Message Board - HealthBoards". http://www.healthboards.com/boards/showthread.php?t=409092. Retrieved 18 July 2011.
- United States Environmental Protection Agency, "IAQ in Large Buildings". Retrieved Jan. 9, 2006.
External links
- Glossary definition of absolute humidity – National Science Digital Library
- Glossary definition of psychrometric tables – National Snow and Ice Data Center
- Glossary definition of specific humidity – National Snow and Ice Data Center
- FREE Humidity & Dewpoint Calculator – Vaisala
- Free Windows Program, Dewpoint Units Conversion Calculator – PhyMetrix
|
|||||||||||||||||